Spin-state dynamics of a photochromic iron(II) complex and its immobilization on oxide surfaces via phenol anchors
Abstract
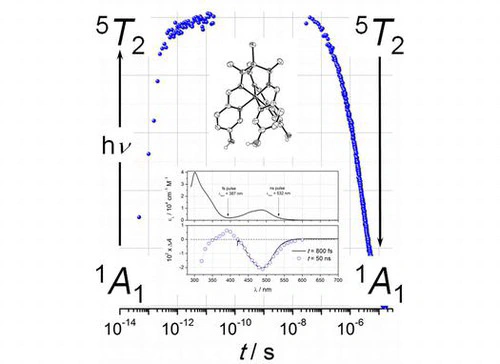
This work presents a detailed study of the photo-induced spin-state dynamics of the photochromic iron(II) complex 1, where the metal ion is in the field of a tripodal hexa-imine ligand with protolysable phenol groups. The nature of the complex’s ground state has been identified as a spin singlet by 1H NMR and steady-state UV/vis spectroscopies, and its distorted octahedral structure was analyzed via crystal structure determination. Sub-picosecond and nanosecond time-resolved laser flash photolysis experiments identify the long-lived quintet state of 1 as the selective product of photoexcitation in the UV/vis spectral region. Thermal barriers of spin-state interconversion as a function of solvent and added base are derived from temperature-dependent rates of transient decay. Ground-state recovery is found to be significantly affected by the solvent and is strongly enhanced, in particular, by base-driven solvolysis of the ligand’s phenol groups. Partial spontaneous deprotonation of the phenolic hydroxyl groups of 1 seems to prevail on metal oxide surfaces, i.e. on alumina. Composite materials, like 1 at Al2O3, that retain the characteristic spectral features of the parent iron(II) complex can be readily obtained by wet impregnation of hydrous alumina with solutions of 1.