Controlled ligand distortion and its consequences for structure, symmetry, conformation and spin-state preferences of iron(II) complexes
Abstract
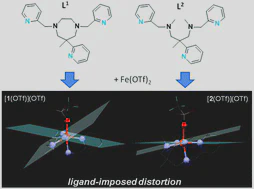
The ligand-field strength in metal complexes of polydentate ligands depends critically on how the ligand backbone places the donor atoms in three-dimensional space. Distortions from regular coordination geometries are often observed. In this work, we study the isolated effect of ligand-sphere distortion by means of two structurally related pentadentate ligands of identical donor set, in the solid state (X-ray diffraction, 57Fe-Mössbauer spectroscopy), in solution (NMR spectroscopy, UV/Vis spectroscopy, conductometry), and with quantum-chemical methods. Crystal structures of hexacoordinate iron(II) and nickel(II) complexes derived from the cyclic ligand L1 (6-methyl-6-(pyridin-2-yl)-1,4-bis(pyridin-2-ylmethyl)-1,4-diazepane) and its open-chain congener L2 (N1,N3,2-trimethyl-2-(pyridine-2-yl)-N1,N3-bis(pyridine-2-ylmethyl)propane-1,3-diamine) reveal distinctly different donor set distortions reflecting the differences in ligand topology. Distortion from regular octahedral geometry is minor for complexes of ligand L2, but becomes significant in the complexes of the cyclic ligand L1, where trans elongation of Fe−N bonds cannot be compensated by the rigid ligand backbone. This provokes trigonal twisting of the ligand field. This distortion causes the metal ion in complexes of L1 to experience a significantly weaker ligand field than in the complexes of L2, which are more regular. The reduced ligand-field strength in complexes of L1 translates into a marked preference for the electronic high-spin state, the emergence of conformational isomers, and massively enhanced lability with respect to ligand exchange and oxidation of the central ion. Accordingly, oxoiron(IV) species derived from L1 and L2 differ in their spectroscopic properties and their chemical reactivity.