Thiocyanate Anchors for Salt-like Iron(II) Complexes on Au(111): Promises and Caveats
Abstract
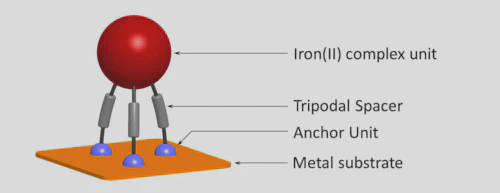
The formation of self-assembled monolayers (SAMs) on Au(111) from solution has been investigated for two ionic iron(II) complexes of the type [Fe(L)](BF4)2, where L is tripodal hexadentate and contains three thiocyanate anchor groups. The ligands (L1, L2; donor set: N6) are obtained by Schiff base condensation of a tripodal triamine (L1: tris-(2-aminoethyl)amine, ‘tren’; L2: 1,1′,1″-trimethyl(thiophosphoryl)trihydrazide) with 5-(4-thiocyanatobutoxy) pyridine-2-carbaldehyde. Layers of the complexes adsorbed on Au(111) from methanol solution have been characterised using scanning tunnelling microscopy (STM), infrared reflectance absorbance (IRRAS), X-ray photoelectron (XPS) and UV/Vis reflectance spectroscopies, as well as ellipsometry. Complex [Fe(L1)](BF4)2 deposits intact on a gold surface and retains its optical addressability. Elaboration of this result may provide access to a new class of self-assembled layers, employing salt-like tripodal coordination compounds with thiocyanate anchors. The second complex, [Fe(L2)](BF4)2, which contains a sulphur atom in the ligand backbone, is not sufficiently stable under the same conditions.