Copper(I) and Iron(II) Complexes of a Novel Tris(pyridyl)ethane-Derived N₄ Ligand: Aspects of Redox Behaviour and Bioinorganic Physicochemistry
Abstract
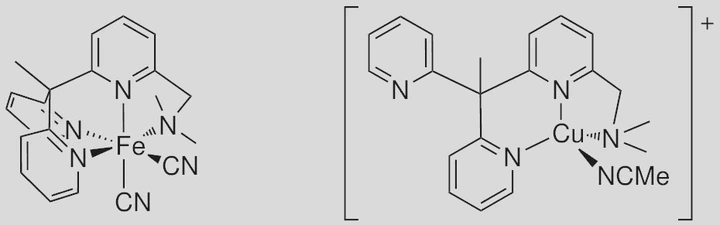
The N4 ligand 1-{6-[1,1-bis(pyridin-2-yl)ethyl]pyridin-2-yl}-N,N-dimethylmethanamine (L) is presented. It has been used to obtain the copper(I) complex [CuL(MeCN)]PF6 and the iron(II) complex [Fe(CN)2L]. Aerobic oxidation of the copper(I) complex is unspecific at room temperature. Anaerobic oxidation in solution, which is very sluggish, occurs with formation of the copper(II) complex [CuIIFL]PF6, suggesting concomitant hydrolysis of hexafluorophosphate initiated by traces of water. The iron(II) complex adsorbed at a TiO2 surface is an efficient photosensitiser of the support. Photoelectrochemical photocurrent switching (PEPS), which can be initiated by changing the electrochemical potential and photon energy, is very pronounced. The construction of a simple photoelectrochemical NOR logic gate has been demonstrated.